Home > Teams > Metabolic and Cardiovascular Diseases > HCEMM-SU Molecular Channelopathies Research Group
Group Leader: László Csanády M.D., Ph.D., D.Sc.
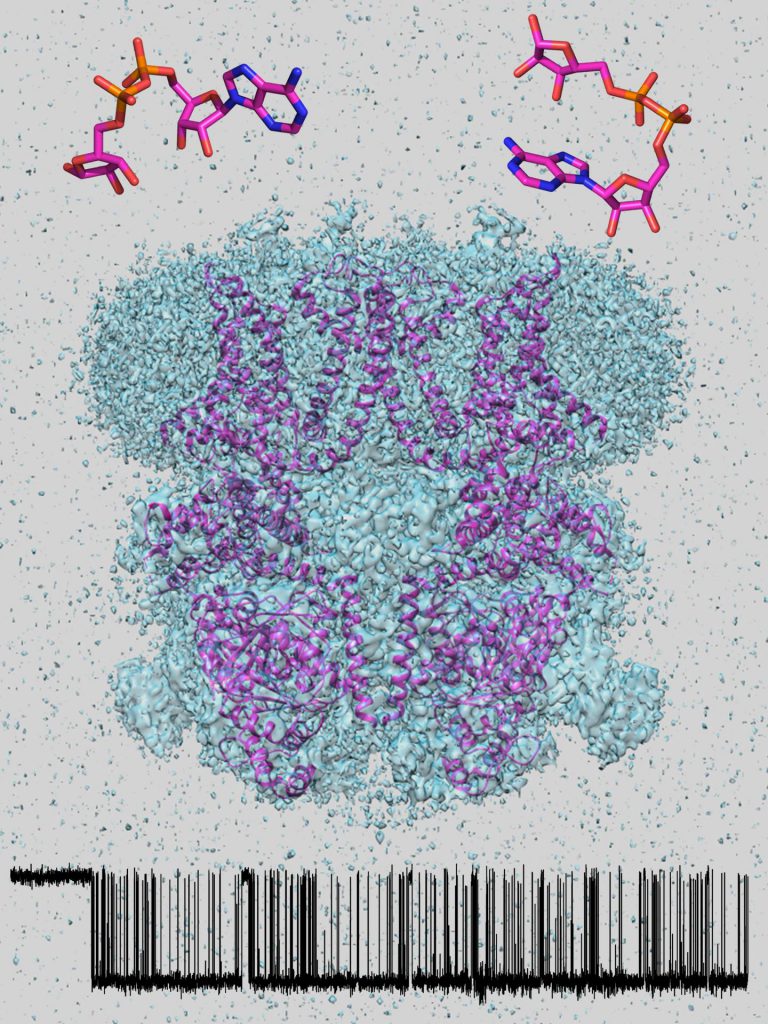
Our group studies structure and function of two medically relevant ion channels, the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) anion channel and the Ca2+ permeable cation channel Transient Receptor Potential Melastatin 2 (TRPM2). CFTR and TRPM2 are known as “channel-enzymes”, proteins in which a single polypeptide chain displays both ion channel and enzymatic properties. Because impaired or excessive activity of either channel causes disease, both are promising therapeutic targets: understanding their molecular mechanisms would be an important conceptual, development of specific activators/inhibitors an outstanding practical, achievement.
CFTR hyperactivity leads to diarrhea (e.g., cholera), whereas its mutations cause cystic fibrosis (CF), the most common lethal inherited disease among whites. A mutation found in 90% of CF patients impairs both CFTR surface expression and channel gating: effective therapy will thus require a combination of a “corrector” drug to enhance surface expression, and of a “potentiator” to stimulate gating. Our goals are to deepen our understanding of CFTR structure, physiological regulation, and the molecular motions that underlie channel gating. We further wish to dissect the molecular pathologies of CFTR mutations that cause CF. Finally, we hope to exploit this knowledge to promote mechanism-guided potentiator design.
TRPM2 activity is needed for the immune response, insulin secretion, and the maintanance of body temperature, but its excessive activation after ischemia causes cell death: thus, both TRPM2 inhibition (stroke, heart attack, Alzheimer’s, inflammation, hyperinsulinism) and stimulation (diabetes, amyotrophic lateral sclerosis, Parkinsonism, bipolar disorder, excessive fever) might become clinically useful. Our aim is to better understand TRPM2 structure, function, and evolutionary adaptation in vertebrates, as well as a detailed pharmacological profiling of the human channel.
Our methodology includes high-resolution electrophysiological (patch-clamp) recordings of channel currents from native cells and heterologous expression systems, in vitro enzymatic assays on heterologously expressed channel proteins purified to homogeneity, electron cryomicroscopy, and in silico structure modeling.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 739593. HCEMM supported by EU Programme: H2020-EU.4.a. – Teaming of excellent research institutions and low performing RDI regions. Project starting date was 1 April 2017.






