Home > Teams > Immuno-inflammatory Diseases > HCEMM-USZ Skin Research Group > Running Projects
The role of epithelial cells in the immunological homeostasis of the skin
The human skin is a complex organ that, in addition to providing a mechanical, chemical and immunological barrier against the external environment, plays an important role in sensation, thermoregulation, and protection from ultraviolet light. Disturbed immunological homeostasis is a characteristic feature of common chronic inflammatory skin diseases, including psoriasis, atopic dermatitis, and acne.
More than 90% of the cells in the epidermis are keratinocytes, which are highly active players of the skin immune system. They express different pattern recognition receptors (PRR), including Toll-like receptors (TLRs), allowing them to recognize conserved molecular patterns, such as pathogen-associated molecular patterns (PAMPs) from pathogenic microbes, as well as danger-associated molecular patterns (DAMPS) present in their environment. Recognition of these signals leads to the activation of a conserved cellular program, including the coordinated change in expression of downstream target genes that play a role in the initiation and execution of innate immune and inflammatory events (1–3). Apart from pathogenic microbes, keratinocytes also recognize members of the cutaneous non-pathogenic, commensal microflora with the same conserved PRR-activating molecular patterns as their pathogenic counterparts, and, thus, may also modify the cellular and molecular properties of the skin. With these properties, skin epithelial cells play important roles in the maintenance of cutaneous homeostasis.
Psoriasis: a disease with disturbed immunological homeostasis
Psoriasis is a chronic, painful, disfiguring and disabling inflammatory skin disease for which there is no cure. It causes great physical, emotional and social discomfort. In 2014, WHO member states recognized psoriasis as a serious non-communicable disease (7). The disease affects ~2–3% of the population (at least 100 million patients worldwide), making psoriasis a serious global problem. Psoriasis primarily involves the skin and the nails but is also associated with a number of comorbidities, such as inflammatory arthritis, cardiovascular diseases, metabolic syndrome, inflammatory bowel disease, and depression.
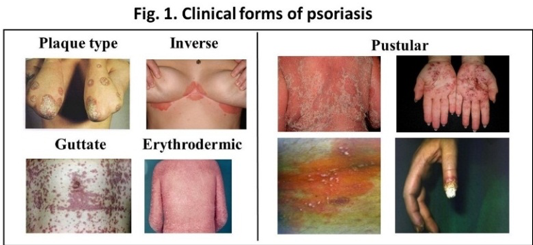
Psoriasis is a complex disease in which multiple genetic factors, together with environmental factors, lead to the appearance of the disease phenotype (8,9). The clinical forms include the plaque-type, guttate, pustular, inverse and erythrodermic psoriasis (Fig. 1). Clinical symptoms usually start later in life, and discordance between identical twins suggests the importance of environmental factors and epigenetic regulatory mechanisms.
Recent studies suggest that susceptibility to psoriasis involves many different cells types, resulting in complex crosstalk among the innate and adaptive immune systems and that both autoimmune and autoinflammatory responses shape clinical presentation (10). Keratinocyte hyperproliferation is a characteristic feature of the disease. The keratinocytes obtained from non-lesional, healthy-looking skin of psoriatic patients exhibit an inherent sensitivity to proliferative signals, and this plays a crucial role in the development of psoriatic skin lesions (11,12). Inherently abnormal immune regulation — both in innate and adaptive responses — is undoubtedly also a key feature in psoriatic pathogenesis and is strongly influenced by the tissue environment. In genetically predisposed individuals, the hyperactive immune response leads to exacerbated inflammation (13). T cells are crucial components in maintaining the disease, and Th17 and Th22 cells and their secreted cytokines, including TNF, IFN-γ, IL-23, IL-17A, IL-17F, and IL-22, play central roles (14–17). During these events, tissue-resident, self-reactive memory cells are also generated, which might later alert inflammatory cells and mediators upon stress, resulting in the development of chronic, recurring inflammatory skin disease (18). Recently, it has been proposed that mesenchymal stem cells (MSCs) are involved in the disturbed tissue and immunological homeostasis in psoriasis; however, their exact roles have not yet been clarified (19). MSCs are able to self-renew and exhibit multilineage differentiation, which allows them to reconstruct functional tissues in vivo. In addition, they can stimulate the activity of surrounding fibroblasts and immune cells. In response to inflammatory stimuli, MSCs inhibit the proliferation of T-lymphocytes, induce M2 macrophage differentiation and facilitate the differentiation and activation of immunosuppressive cells for further control of inflammatory processes through direct and indirect pathways. Therefore, cell therapy with MSCs might represent a new way in psoriasis therapy.
Although systemic immune activation is believed to play a key role in the induction of psoriasis, psoriatic lesions tend to recur at the same locations (for example, on the knees and elbows or, in inverse psoriasis, in the folds of the skin). This observation suggests that, in addition to systemic cytokine-storm factors, the local tissue environment mediated resident cells, such as keratinocytes, play a role in this phenomenon. However, the exact mechanism leading to the reoccurring development of psoriasis at the same location has not yet been clarified.
Our previous results on the role of keratinocytes in skin immune system and in inflammatory skin diseases.
Psoriatic keratinocytes from uninvolved skin exhibit many functional abnormalities and might play an important role in the early steps of the disease. Activated T cells are important components of the inflammatory infiltrate in psoriatic lesions (11). Lesional CD4+ T cells produce a mixture of lymphokines that stimulate the growth of psoriatic non-lesional basal keratinocytes. In vitro recombinant interferon-γ in the presence of granulocyte-macrophage-colony-stimulating factor (GM-CSF) and interleukin (IL) 3 mimics this effect, and D-type cyclins play important roles in the regulation of excessive basal-cell proliferation and perturbed keratinocyte differentiation in the psoriatic epidermis (20). To gain deeper insight into the functional differences of healthy and psoriatic non-lesional keratinocytes, we performed several large-scale studies and identified genes that were differentially expressed in a basal state or following in vitro treatment with T-cell lymphokine (21–24). One of the molecules we studied extensively in the past few years is the non-coding RNA, PRINS, which is differentially expressed in psoriatic non-lesional and healthy epidermis and plays a key role in the stress response of keratinocytes (21,25). PRINS regulates the expression of the G1P3 protein, playing important roles in the regulation of keratinocyte proliferation and apoptosis (26), and participates in direct physical interaction with nucleophosmin and GRP94. Together these proteins form a complex, which regulates important pathogenic events during lesion formation, including cellular stress response, keratinocyte proliferation, and differentiation (27). Extracellular matrix (ECM) defects have also been observed in the psoriatic non-lesional skin. We found several ECM components, including laminin, a5b1 integrin and extra domain-A containing fibronectin (EDA+FN), that were abnormally present around basal keratinocytes, and the expression of keratinocyte growth factor and its receptor were also increased, compared to healthy controls (23,28).
Innate immune activation has a complex role in psoriasis pathogenesis. Epidermal keratinocytes sense the cutaneous microbiota with PRRs. In past years, we intensively studied the molecular events initiated in these cells in response to a prominent member of the cutaneous microbiota, Cutibacterium acnes (C. acnes). Recognition of this bacterium is mostly mediated by the activation of TLR-2 and TLR-4, resulting in induction of the canonical signaling pathway and subsequent innate immune and inflammatory events (1–3). Recently, we identified TNF Alpha-Induced Protein 3 (TNFAIP3) and TNFAIP3 interacting protein 1 as potent regulators of TLRs-induced inflammatory cascades. Both proteins may regulate C. acnes-induced signaling events through the establishment of a negative regulatory feedback loop controlling NF-κB activity in keratinocytes (6,29). Both molecules have been linked recently to psoriasis pathogenesis (30). In addition to PAMPs, cytosolic nucleotide fragments act as pathogen- or self-derived trigger factors (DAMPs), induce innate immune and inflammatory reactions, and enhance chronic inflammation by activating inflammasomes and the expression of inflammatory cytokines (31). A potential stress signal in the involved psoriatic skin may be extracellular DNA, which activates the AIM2 inflammasome resulting in the cleavage of the proIL-1β cytokine into functioning IL-1β, and induces the expression of several inflammatory cytokines in professional immune cells (32).
Recently, it has been shown that inflammasome activation in mouse epithelial stem cells via the AIM2-caspase-1-interleukin-1 pathway results in the development of “inflammatory memory” of the epithelial cells (33), and the mechanism of inflammatory memory was shown to involve epigenetic changes analogous to those recently described for innate immune memory (34). We found recently that expression of caspase recruitment domain family member 18 (CARD18, Iceberg), a known negative regulatory molecule that inhibits inflammatory events by terminating inflammasome activation due to direct interaction with procaspase-1, differs in the healthy and psoriatic skin. Furthermore, CARD18 showed an altered response to inflammatory stimuli (lymphokine treatment or mechanical injury) in the healthy and psoriatic skin (9). As inflammasome activation is critical in the development of “inflammatory memory” of epithelial cells, the abnormal activation of the inflammasome pathway might result in an abnormal “inflammatory memory” in psoriasis.
Hypothesis 1:
We hypothesize that repeated exposure of the skin to PAMPs and/or DAMPs is usually beneficial for the skin, that results in temporarily disturbed homeostasis, but in most cases, the disturbed homeostasis returns to a normal state, and providing improved fitness of the skin. We hypothesize that this improved fitness might be due to the memory of previous exposure, resulting in better protection or tolerance to microbes, and improved skin barrier. We refer to this phenomenon as “good memory.”
Research questions to be answered:
How do keratinocytes respond to repeated exposure to PAMPs and DAMPs? Do psoriatic keratinocytes respond to these environmental stimuli differently? After repeated exposure, what kind of regulatory mechanisms are responsible for the development of tolerance or for the increased activation of a special cell type?
Hypothesis 2:
We propose that, in psoriasis, chronic mechanical injury on the extremities or recurrent microbial infections in the folds result in an increased sensitivity of keratinocyte stem cells to systemic inflammatory signals, and this increased sensitivity results in the recurrence of the lesions at the same location. The lack of the development of local tolerance and/or increased sensitivity to previous inflammatory signals results in the permanent inflammatory conditions seen in psoriasis. Keratinocytes differentiated from hiPSCs from psoriatic patients might serve as a new in vitro disease model for psoriasis. The abnormal immunological homeostasis in psoriasis might be normalized with MSCs or with early new treatments.
Research questions to be answered:
Are there molecular and cellular differences in the cellular compartments of the skin of patients with psoriasis, in particular between clinically noninvolved skin that has never exhibited psoriasis and noninvolved skin that has exhibited psoriasis (resolved skin)? Do keratinocytes obtained from clinically noninvolved skin, that has never exhibited psoriasis respond differently to immunological activating signals than keratinocytes from resolved skin? How psoriatic hiPSC-derived keratinocytes respond to proinflammatory stimuli? Is it possible to down-regulate the increased sensitivity of psoriatic keratinocytes to immune activation by adipose-derived stem cells that exert immunosuppressive properties? Can we modulate the local tissue memory in psoriasis with early treatment and/or with novel medications identified using the drug-repurposing approach?
Objectives:
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 739593. HCEMM supported by EU Programme: H2020-EU.4.a. – Teaming of excellent research institutions and low performing RDI regions. Project starting date was 1 April 2017.






